Home
Lab Tour
Lab Syllabus
Papers
Symposium
 Resources Resources
-
-
|
-

Lab 5: Independent Investigation of Neuromuscular Physiology
[Reading] [Overview] [Useful Links] [Paper and Talk] [Further Study]
Pre-lab reading
- Lab manual - pages 49-52
- If someone in your lab group has a copy of a human anatomy textbook, bring it to lab this week to look up muscles you might want to record from.
Overview
In this lab you will use the techniques you learned in the previous two labs, Stimulating Skeletal Muscle and Electromyography, to conduct an investigation of your own. In addition to the materials from those two labs, additional equipment will be provided to allow a wider range of topics (see the lab tour page). You can choose some type of neuromuscular phenomenon or activity, devise questions you want to answer about nerve or muscle function, and choose which techniques to employ. Your lab group must submit a 1-2 page written proposal in advance of the lab (according to the deadline set by your GSI). The proposal should include (1) the question(s) you want to investigate, (2) your hypotheses (including predicted results AND an explanation of why you expect them), (3) a complete description of what the subject(s) will do and what parameters you will monitor, including the number of trials of each treatment. The proposal may be submitted on paper or as an e-mail to your GSI. Note that only one proposal should be submitted for each group, and all members will receive the same grade. The proposal will be reviewed by your GSI, who will suggest improvements and make sure that the necessary equipment and supplies are available for you next week.
Project Ideas
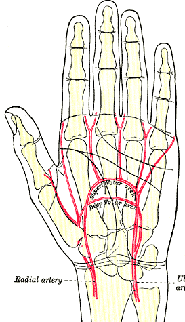
- Compare responses to electrical stimulus or EMGs of muscles that differ in fiber types. The superficial digital flexor has a fairly even mix of fast glycolytic, fast oxidative, and slow oxidative fibers. For a muscle with predominantly slow oxidative fibers, you could try the adductor polices, a muscle that adducts the thumb and can be stimulated via the ulnar nerve at the wrist. One muscle with mostly fast fibers is the first dorsal interosseus, which can be stimulated by a motor point between the thumb and 2nd digit.
- How might muscle perfusion affect time to fatigue or recovery from fatigue as measured with the Physiogrip? One manipulation you could try is to cut off blood flow to the arm by inflating a sphygmomanometer (blood pressure cuff) on your upper arm to a pressure higher than your systolic blood pressure. This will effectively eliminate arterial supply and venous return to the muscles of the forearm and hand. To avoid complete numbness and discomfort we suggest a 5-minute limit to cuff inflation.
- How does the firing pattern change when a muscle becomes fatigued? Are the smaller motor units that are used for fine control affected? Does fatigue caused by maximal voluntary contraction affect the ability to perform finely-controlled small contractions? How does the frequency spectrum of MUAPs change with muscle force and fatigue?
- What muscles are active in maintaining posture? Are they the same ones that are active in causing joint movements in walking or running? How do different postures affect the EMG activity of postural muscles?
- Compare the role of a muscle in different activities. We have treadmills, cycle ergometers, and steps. The treadmills can be tilted to simulate going uphill and downhill.
- Some muscles span two joints. How they are used when only one joint moves or when the two joints move in opposite directions (e.g. one flexes while the other extends).
- What is the relative importance of concentric and eccentric contractions of a muscle in various activities and does the EMG differ?
- Does nerve conduction velocity vary with temperature? Extremities can be chilled in water to test this.
- Do all motor neurons exhibit the same conduction velocity? Another way to study nerve conduction is by combining evoked potentials with EMGs. If you stimulate a somatic nerve electrically at two different places while recording an EMG from one of the muscles it innervates, then the difference between locations in the time it takes from stimulus to response represents the conduction time between the two locations.
- If you measure the conduction velocity of a motor nerve as in the previous project idea, then you can calculate when the muscle should have responded to the stimulus if the signal traveled directly to the electrode without slowing or delay. The difference between the predicted and actual time of muscle response is the residual latency. What is the residual latency? What does it represent? Does it vary?
- When a person learns a challenging new activity that requires good motor control, does the pattern of EMGs change as skill improves? Do EMGs of highly skilled subjects differ from those of unskilled ones?
- How do forearm muscles function in typing on a computer keyboard? Does keyboard height or the use of a wrist rest affect EMG activity?
- If you want to design a myoelectric prosthetic arm for a person with amputation below the elbow, what muscles would be best to use and where should the electrodes be placed? (A myoelectric prosthesis uses the electrical activity of remaining limb muscles to drive motors that move joints in the artificial limb.)
- How do voluntary reaction times differ for stimuli of various modes (visual, touch, auditory), location of stimulus (for touch), and place where response is delivered (hand, foot)? What roles do neural pathway lengths and nerve conduction velocities play in any differences that exist? Are reflex reactions any faster or slower than voluntary reactions along similar neural pathways?
To see pictures of the equipment and how it is used, visit the
lab tour page.
Useful Links
Paper and Oral Presentation
There is no group lab report due for this lab. Instead, some group members will write a paper on this investigation and the entire group will collaborate on an oral presentation to be delivered in the class symposium. See the Papers and Symposium web pages for more details.
Further Study at Berkeley
Courses related to this lab topic
IB124. Musculoskeletal Biomechanics. The purpose of this course is to teach you how to analyze the musculoskeletal system in humans and other animals using the principles of physics and physiology.
IB125. Locomotion Biomechanics. This course provides a quantitative and integrative analysis of human locomotion. Topics progress from a review of anatomical structures to the muscle activity patterns, quantification of movements (kinematics), forces and torques (kinetics), mechanical and metabolic energy involved in normal human walking and running. Additional topics include the evolution of locomotion, locomotion of quadrupeds and other vertebrate animals, and legged robots.
IB126. Neuromuscular Fatigue. Analysis of mechanisms of nerve and muscle excitation and muscle contraction, and changes occurring during fatigue and recovery.
IB127. Motor Control. We will develop a basic understanding of modern theories of information and control, then analyze neuromotor systems to understand the elements of motor control systems; muscles, sensory transducers and motor systems of the brain. We will use information and control theories to synthesize knowledge of the elements into understanding of the control systems that regulate posture, locomotion, and voluntary movement.
Faculty doing research related to this lab:
- Robert J. Full (IB) Comparative biomechanics, physiology, and functional morphology (PolyPEDAL lab - very cool site!)
- Steven L. Lehman (IB) Motor Control
-
-
Links on these pages to commercial sites do not represent endorsement by the University of California or its affiliates.
-
IB 132 Lecture Website
Department of Integrative Biology
U. C. Berkeley
-
-
-
Last updated 1/18/05
- Copyright © Department of Integrative Biology. All rights reserved 2005
-
-
-
|
|